- Artikel
- Bron: Campus Sanofi
- 10 jul 2025
Expert-presentatie: de impact van type-2-inflammatie bij COPD

My name is Surya Bhatt. I'm a professor of Medicine at the University of Alabama at Birmingham and I'm a COPD researcher. This talk is sponsored by Sanofi and Regeneron and you won't get any (CME) credit. In response to any kind of insult. For example, cigarette smoke, viral infections, bacterial infections, pollution, there is some element of epithelial injury and epithelial damage. And the first response to this usually is once there is enough epithelial damage, there is release of damage associated molecular patterns or (DAMPs) which are now perhaps more popularly called alarmins and these are IL-33, IL-25 and TSLP or thymic stromal lymphopoietin. So all these can be released and they all have specific roles in terms of furthering inflammation. And IL-33 is perhaps best understood and some people consider it the master inflammation switch where once it's activated, it stays activated for a long time. It promotes both type 1 and type 3 inflammation as well as type 2 inflammation. The traditional thinking has been that the prime driver of type two inflammation is IL-5, which causes eosinophilic inflammation. But now we understand that both IL-4 and IL-13 are important in driving type 2 inflammation. And here's a list of all the things that they can do.
lL-4 and IL-13:
- Can both cause epithelial barrier disruption.
- They can cause B cell class switching and enhance an increased production of IgE
- They can cause mast cell degranulation
- They can cause basophil degranulation
- And also eosinophilic inflammation just like IL-5.
- They can also result in goblet cell hyperplasia and mucus production especially IL-13.
And as a consequence of these, they can cause significant airway remodeling as well as alveolar destruction.
IL-4 and IL-13 levels are also elevated during an acute exacerbation as opposed to in the stable state or patients without COPD. So this is also important to note that these pathways are activated in patients with acute exacerbation of COPD and they present and important in COPD.
And then, you know, we all know that there's been a lot of understanding of mucin and mucus production in COPD. And one of the prime drivers of that is MUC5A and IL-4 can induce increased expression of MUC5AC in human epithelial cells. And there's also a dose dependent response almost of IL-13 on MUC5A as well as increased mucus production. So, especially IL-13 has been found to be an important driver of mucus production in patients with COPD.
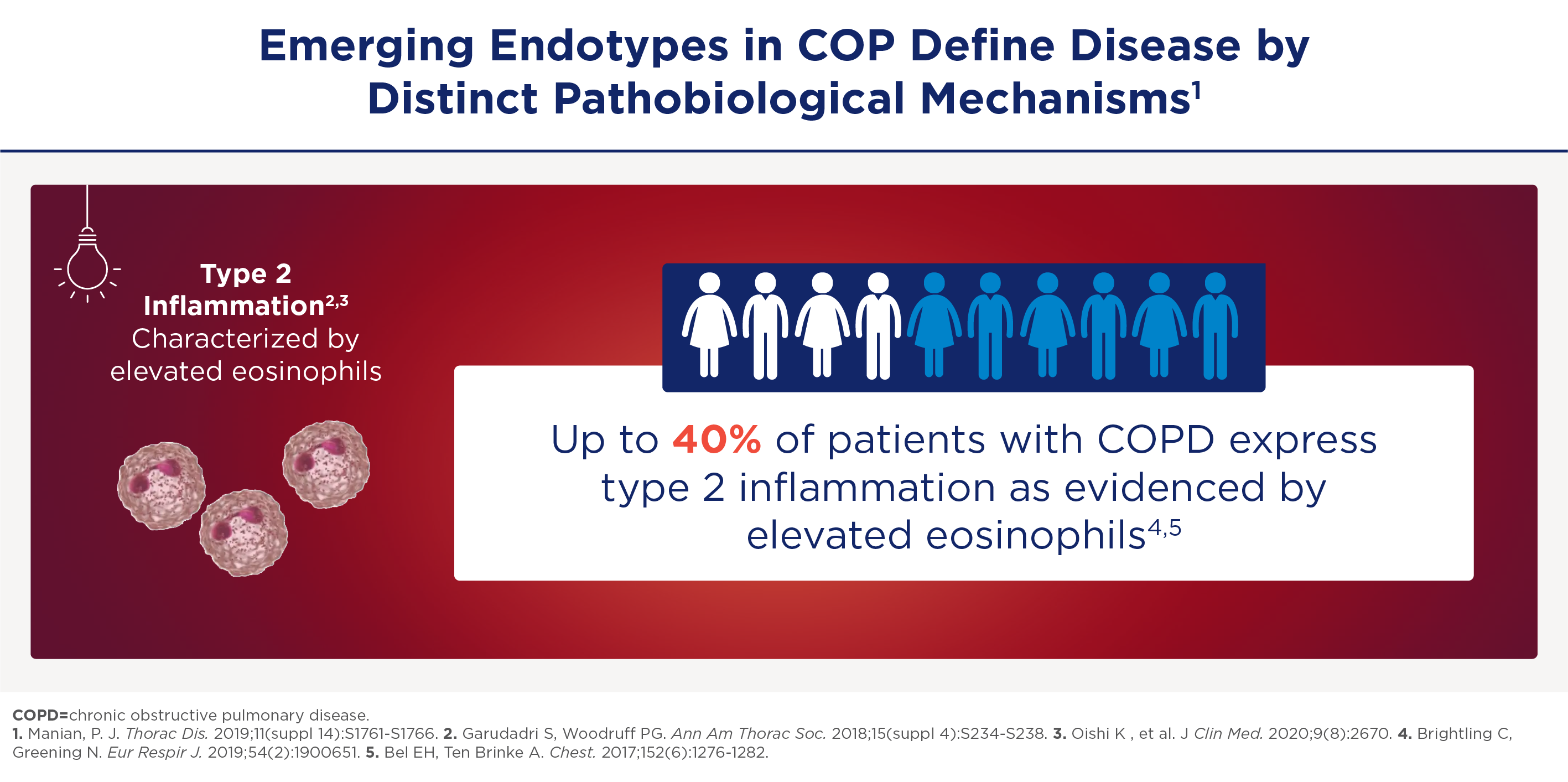
COPD kent neutrofiele én eosinofiele ontstekingsroutes
COPD wordt voornamelijk veroorzaakt door de neutrofiele Th1- en Th17-routes. Dat is belangrijk, maar dat zijn niet de enige routes. Zo is er steeds meer erkenning dat ook eosinofiel-gedreven type-2-inflammatie een rol kan spelen bij 30-40% van de COPD-patiënten.
Een belangrijke biomarker voor het detecteren van type 2-inflammatie is het eosinofielen-aantal in het bloed (BEC). Volgens het GOLD-Report 2025 correleert dat goed met eosinofiele ontsteking in de longen. Omdat de BEC kan fluctueren is frequent bepalen belangrijk. Lees hier meer over BEC en inflammatie-type-2.
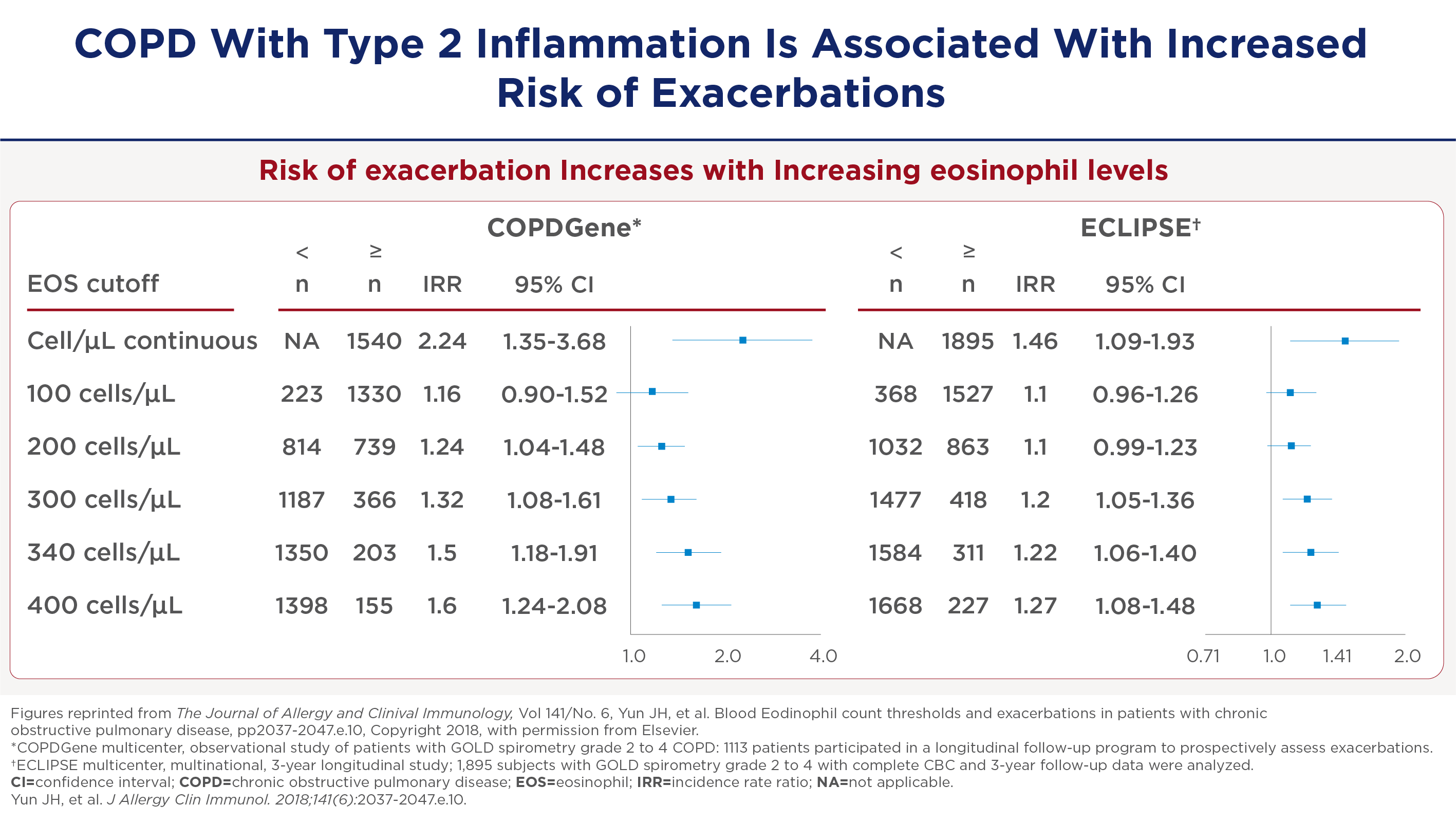
COPD met type-2-inflammatie geeft vaker exacerbatie en heropname in het ziekenhuis
Studies zoals de COPD Gene en Eclipse tonen een duidelijke correlatie tussen het aantal bloed-eosinofielen en de frequentie van exacerbaties. Vanaf 300 cellen per microliter is er een significante toename in de frequentie van exacerbaties, die blijft toenemen bij hogere waarden.
Toename van het aantal ernstige exacerbaties geeft ook meer heropnames in het ziekenhuis. Deze bevinding onderstreept het belang van het monitoren van eosinofielenwaarden voor het voorspellen van zowel matige als ernstige exacerbaties. Zo kunnen behandelstrategieën daarop worden afgestemd om heropnames in het ziekenhuis te verminderen.
* De referenties en de bovenstaande inhoud zijn gebaseerd op de presentatie van Dr. Bhatt; zie de video voor meer informatie.
Samenvatting
Professor Surya Bhatt benadrukt de betrokkenheid van type-2-inflammatie bij een grote groep COPD-patiënten. Dit impliceert een significante verschuiving die kan leiden tot een meer gepersonaliseerde behandelmethode voor COPD: het frequent meten van het eosinofielen-aantal in het bloed en het inzetten van medicatie met een nieuw aangrijpingspunt.
-
Gandhi NA, Bennett BL, Graham NMH, et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov. 2016;15(1):35-50.
-
Yousuf A, Ibrahim W, Greening NJ, et al. T2 Biologics for Chronic Obstructive Pulmonary Disease. J Allergy Clin Immunol Pract. 2019;7(5):1405-1416.
-
Aghapour M, Raee P, Moghaddam SJ, et al. Airway Epithelial Barrier Dysfunction in Chronic Obstructive Pulmonary Disease: Role of Cigarette Smoke Exposure. Am J Respir Cell Mol Biol. 2018;58(2):157-169.
-
Barnes JP. Inflammatory endotypes in COPD. Allergy. 2019;74(7):1249-1256.
-
Wang Z, Bafadhel M, Haldar K, et al. Lung microbiome dynamics in COPD exacerbations. Eur Respir J. 2016;47(4):1082-1092.
-
Smithgall MD, Comeau MR, Yoon BRP, et al. IL-33 amplifies both Th1- and Th2-type responses through its activity on human basophils, allergen-reactive Th2 cells, iNKT and NK cells. Int Immunol. 2008;20(8):1019-1030.
-
Senra L, Mylonas A, Kavanagh RD, et al. IL-17E (IL-25) enhances innate immune responses during skin inflammation. J Invest Dermatol. 2019;139(8):1732-1742.
-
Linden D, Guo-Parke H, Coyle PV, et al. Respiratory viral infection: a potential “missing link” in the pathogenesis of COPD. Eur Respir Rev. 2019; 28(151):180063.
-
Global Initiative for Chronic Obstructive Lung Disease (GOLD). Accessed July 27, 2023. https://goldcopd.org/2023-gold-report-2/.
-
Calderon AA, Dimond C, Choy DF, et al. Targeting interleukin-33 and thymic stromal lymphopoietin pathways for novel pulmonary therapeutics in asthma and COPD. Eur Respir Rev. 2023;32(167):220144.
-
Kurokawa M, Matsukura S, Kawaguchi M, et al. Interleukin-33-activated dendritic cells induce the production of thymus and activation-regulated chemokine and macrophage-derived chemokine. Int Arch Allergy Immunol. 2013;161(Suppl 2):52-57.
-
Griesenauer B, Paczesny S. The ST2/IL-33 axis in immune cells during inflammatory diseases. Front Immunol. 2017;8:475.
-
Borowczyk J, Shutova M, Brembilla NC, et al. IL-25 (IL-17E) in epithelial immunology and pathophysiology. J Allergy Clin Immunol. 2021;148(1):40-52.
-
Claudio E, Wang H, Kamenyeva O, et al. IL-25 orchestrates activation of Th cells via conventional dendritic cells in tissue to exacerbate chronic house dust mite–induced asthma pathology. J Immunol. 2019;203(8)2319-2327.
-
Kotlyarov S. Involvement of the innate immune system in the pathogenesis of chronic obstructive pulmonary disease. Int J Mol Sci. 2022;23(2):985.
-
Gandhi NA, Pirozzi G, Graham NMH. Commonality of the IL-4/IL-13 pathway in atopic diseases. Expert Rev Clin Immunol. 2017;13(5):425-437.
-
Saatian B, Rezaee F, Desando S, et al. Interleukin-4 and interleukin-13 cause barrier dysfunction in human airway epithelial cells. Tissue Barriers. 2013;1(2):e24333.
-
Zheng T, Zhu Z, Wang Z, et al. Inducible targeting of IL-13 to the adult lung causes matrix metalloproteinase- and cathepsin-dependent emphysema. J Clin Invest. 2000;106(9):1081-1093.
-
Zhu Z, Homer RJ, Wang Z, et al. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersecretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103(6):779-788.
-
Alevy YG, Patel AC, Romero AG, et al. IL-13-induced airway mucus production is attenuated by MAPK13 inhibition. J Clin Invest. 2012;122(12):4555-4568.
-
Wang X, Xu C, Ji J, et al. IL-4/IL-13 upregulates Sonic hedgehog expression to induce allergic airway epithelial remodeling. Am J Physiol Lung Cell Mol Physiol. 2020;318(5):L888-L899.
-
Cooper PR, Poll CT, Barnes PJ, et al. Involvement of IL-13 in tobacco smoke-induced changes in the structure and function of rat intrapulmonary airways. Am J Respir Cell Mol Biol. 2010;43(2):220-226.
-
Doyle AD, Mukherjee M, LeSuer WE, et al. Eosinophil-derived IL-13 promotes emphysema. Eur Respir J. 2019;53(5):1801291.
-
Sun J, Liu T, Yan Y, et al. The role of Th1/Th2 cytokines played in regulation of specific CD4+ Th1 cell conversion and activation during inflammatory reaction of chronic obstructive pulmonary disease. Scand J Immunol. 2018;88(1):e12674.
-
Kim CH, Kim KE, Yoon JH, et al. Upregulation of MUC5AC gene expression by IL-4 through CREB in Human airway epithelial cells. J Cell Biochem. 2009;108(4):974-981.
-
Yu H, Li Q, Kolosov VP, et al. Interleukin-13 induces mucin 5AC production involving STAT6/SPDEF in human airway epithelial cells. Cell Commun Adhes. 2010;17(4-6):83-92.
Neem contact op

MAT-NL-2500425