- Artikel
- Bron: Campus Sanofi
- 15 jul 2024
Detectability of Autoimmune Type 1 Diabetes

Today, individuals with autoimmune Type 1 Diabetes (T1D) are often diagnosed reactively when symptoms and/or acute complications become apparent.
Screening for islet autoantibodies may enable early detection of autoimmune T1D in the pre-symptomatic stages of the condition’s progression.
Early detection can help prevent that individuals with autoimmune T1D experience a traumatic diabetic ketoacidosis (DKA) event when they progress to symptomatic Stage 3 of their condition.6-9 Early detection in due time before people reach Stage 3 may allow for a softer and safer transition into life with chronic autoimmune T1D.2,10
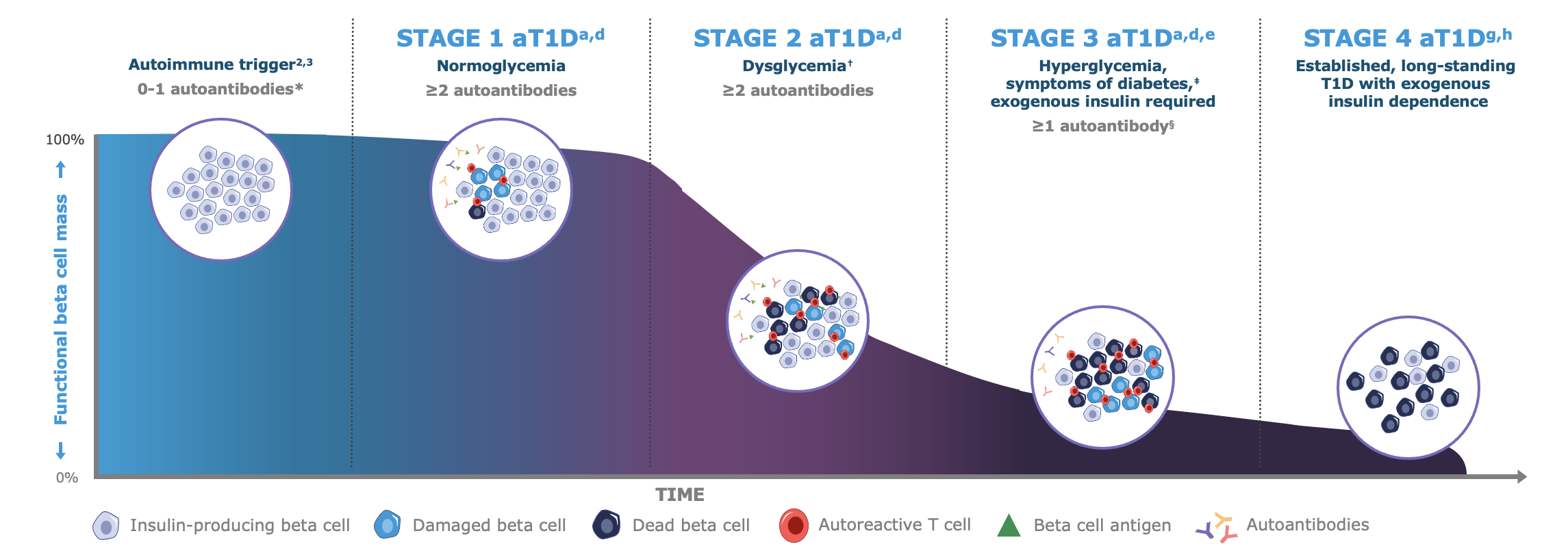
* Autoantibodies to ≤1 beta cell autoantigen (insulin, glutamic acid decarboxylase [GAD65], islet antigen 2 [IA-2], or zinc transporter 8 [ZnT8]) are detected in patient serum.a †Fasting plasma glucose (FPG) of 100 to 125 mg/dL or 2-hour plasma glucose (PG) during oral glucose tolerance test (OGTT) of 140 to 199 mg/dL, or A1C 5.7%-6.4% (39-47 mmol/mol) or ≥10% increase in A1C is observed.d ‡Common symptoms of T1D include polydipsia, polyuria, hunger, extreme fatigue, blurry vision, and weight loss.a,d §In some patients, autoantibodies may become absent in Stage 3 T1D.d
Note: Figure is an example for illustrative purposes, drawn up by Sanofi, based on ref. a-g:
a. Insel RA et al. Diabetes Care (2015) 38 1964–74 | b. van Belle TL et al. Physiole Rev 2011; 91: 79–118 | c. Jacobsen LM, et al. Front Endocrinol (Lausanne). 2018 9,70 | d. American Diabetes Association Professional Practice Committee. Diabetes Care 2022; 45 (Suppl. S1): S17–S38 | e. McCall AL & Farhy LS. Minerva Endocrinol 2013; 38: 145–63 | f. Mayo Clinic. Diabetes symptoms when diabetes symptoms are a concern https //www.mayoclinic.org/diseases-conditions/diabetes/in-depth/diabetes-symptoms/art-20044248 (Accessed 15 Sep. 2025) | g. Sims EK, et al. (2022) Diabetes 71(4) 610-623 | h. Besser REJ, et al. (2022) Pediatr Diabetes. 23(8) 1175-1187.
Who Can Benefit from Early Detection of Autoimmune T1D?
In most countries worldwide, the incidence rate of autoimmune T1D is growing. By 2040, up to ~17.4 million people globally will be diagnosed with this condition.1,11,13,14,16-18
Regardless of their family history, people from all backgrounds and all ages are at risk of developing autoimmune T1D, and although genetic factors may play a role in development of autoimmune T1D, it is found that ~90% of people who will receive a diagnosis do not know of a family member who has also been diagnosed with autoimmune T1D.19 Additionally, up to 38% of autoimmune T1D cases develop in children and young people under the age of 20,16 which challenges the notion that Type 1 Diabetes is a condition that mostly affects children and adolescents.
What Are the Risks of Autoimmune T1D Progressing Undetected?
Autoimmune Type 1 Diabetes quietly destroys beta-cell function. This destruction occurs in three stages, progressively developing from pre-symptomatic Stages 1 and 2 into Stage 3 where the more commonly known symptoms of Type 1 Diabetes may manifest due to hyperglycemia.1,11,14,15














Note: Illustrations produced by Sanofi with data adapted from image references a-j.
a) Moede T et al. (2020) Diabetologie vol. 63 2064–75
b) van Belle T, et al. (2011) Physiol Rev. 91(1) 79-118
c) Tosur M et al. (2020) BMJ Open Diabetes Res Care 8 e000801
d) Insel RA et al. (2015) Diabetes Care. 38(10) 1964-1974
e) Pugliese A. (2017) Journal of Clin. Invest. vol. 127, no. 8 pp2881–91
f) Rodriguez-Calvo T et al. (2021) Front Immunol vol. 12 article 667989
g) Burrack AL, et al. (2017) Front Endocrinol (Lausanne) 8343
h) American Diabetes Association Professional Practice Committee. (2022) Diabetes Care. 45(suppl 1) S17-S38
i) McCall AL & Farhy LS. (2013) Minerva Endocrinol. 38(2) 145-163
j) Mayo Clinic. Diabetes symptoms when diabetes symptoms are a concern https //www.mayoclinic.org/diseases-conditions/diabetes/in-depth/diabetes-symptoms/art-20044248 (Accessed 02 May 2024)
People with autoimmune T1D who go undetected until they progress to Stage 3 have an increased risk of experiencing diabetic ketoacidosis (DKA). DKA events are potentially life-threatening complications that could lead to long-term negative outcomes.3,20
DKA at diagnosis of autoimmune T1D in children predicts poor long-term glycemic control, independent of demographic and socioeconomic factors.20
Anyone who in the long-term deviates from blood glucose levels (according to guidelines) risk experiencing other serious and/or potentially life-threatening complications, including:17,20-26
- Eye damage (retinopathy)
- Nerve damage (neuropathy)
- Foot complications (sores and blisters)
- Kidney disease (nephropathy)
- Increased risk of heart- and artery disease
- Sub-clinical brain alterations and detrimental neurocognitive outcomes
- Increased morbidity and mortality
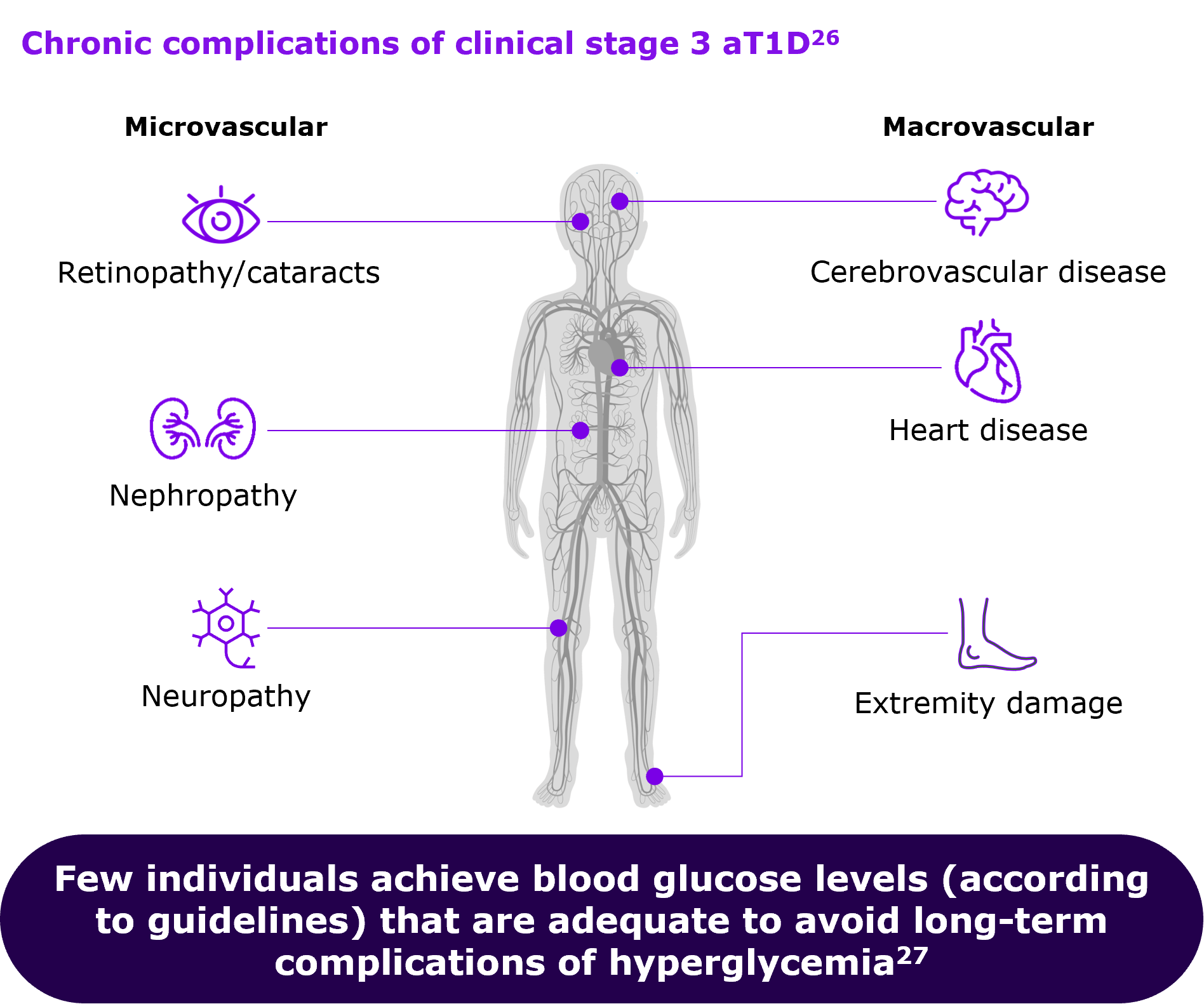
Figure drawn up by Sanofi, based on ref.26,27
Biomarkers to ‘Predict’ Type 1 Diabetes during Pre-Symptomatic Stages
Due to the insidious progression through the pre-symptomatic Stages 1 and 2, autoimmune T1D is hardly detectable by more conventional markers such as hyperglycemic blood glucose levels and associated clinical symptoms like extreme thirst (polydipsia), excessive urination (polyuria), unexpected weight loss, unusual fatigue, or disturbances of vision.15,21
It is possible to reliably identify individuals who are progressing through Stages 1 and 2 of autoimmune T1D through blood tests for diabetes-specific autoantibodies, which target proteins associated with secretory granules in the beta cells, serving as biomarkers of T1D-associated autoimmunity.1,11,28-31
The primary autoantibodies involved in autoimmune T1D include glutamic acid decarboxylase antibodies (GADA), insulinoma-associated-2 autoantibodies (IA-2A), insulin autoantibodies (IAA), and zinc transporter 8 autoantibodies (ZnT8A), with potential repetitive testing based on the result.1,18,31
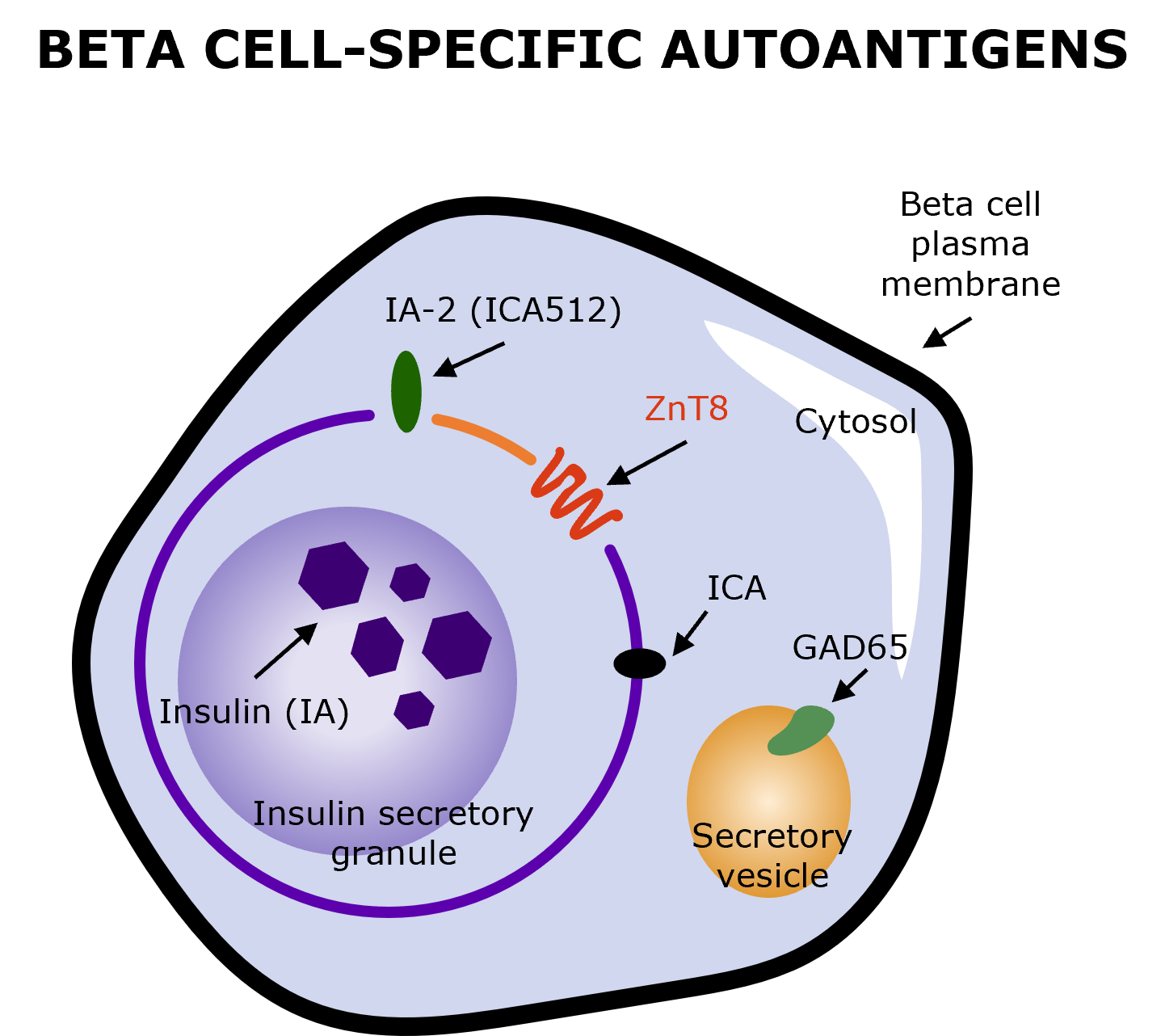
Illustration produced by Sanofi with data from Arvan P, et al. (2012) (Fig. 2)31
The number of detectable autoantibodies through screening correlates with the progression of autoimmune T1D.11 In children, the conversion to having two or more autoantibodies is linked with an 84% risk of developing Stage 3 autoimmune T1D by the age of 18.32
Overall, age can be a predictive biomarker for autoimmune T1D because of peak in the incidence of islet autoantibody seroconversion before the age of 3.4,33-35 As such, the risk of a 1-year-old child developing islet autoantibodies is greater than that of a 5-year-old child or when (s)he reaches 5 years of age without developing islet autoantibodies.36
The biomarkers to detect autoimmune T1D change with age, making prediction in adults very different from prediction in children.36 Adults who develop the condition are likely to only present with the GADA biomarkers.37

Why Consider Early Detection through Screening for Islet Autoantibodies?
Because Stages 1 and 2 are pre-symptomatic, the development of autoimmune T1D may go undetected for months or even years,13,14 leaving those affected – and potentially their families and caregivers – blindsided by symptoms onset and by the shock of diagnosis.10,38,39
Early detection of biomarkers and identification of people who are islet autoantibody-positive (IAb+) can provide an opportunity for people to become familiar with the effects that autoimmune T1D will have on their daily lives – even before symptoms may manifest.10,38,39
It has been shown that children who had been diagnosed with pre-symptomatic autoimmune T1D as part of a screening program had milder clinical presentation at diagnosis, compared to children diagnosed at the symptomatic Stage 3 and who had not been screened for islet autoantibodies.40,41 A potentially milder clinical presentation can show as follows:41
- lower HbA1c and fasting blood glucose levels
- higher fasting C-peptide levels
- fewer children with ketonuria
- fewer children requiring insulin at diagnosis
- low prevalence of DKA
- normal BMI-SDS, possibly reflecting less weight loss
Achieving fasting blood glucose values that are in accordance with guidelines is associated with lower risk of kidney and cardiovascular complications.42 Moreover, good C-peptide preservation has been associated with a lower frequency of severe hypoglycemic episodes and a lower incidence of diabetes-related ophthalmological disorders, e.g. retinopathy.43,44
While being monitored for progression and the almost-inevitable onset of insulin-dependent Stage 3 T1D, those who are at risk and living with early stage autoimmune T1D (both single or multiple IAb+) have a significantly reduced risk of experiencing DKA at diagnosis.6,41
Early detection also provides opportunities for the affected and healthcare professionals to structure education and training, aimed to assist in successfully managing the psychosocial impacts of being diagnosed with a chronic condition and to enable a gentler beginning to life with autoimmune T1D.6,10,38,39
The 1st Decision is about
who to screen
and how to screen
Sanofi is committed to providing a new understanding and perspective on autoimmune Type 1 Diabetes. Join us!
Neem contact op

Referenties
-
ElSayed NA et al. (2023) Diabetes Care 46 (Suppl. 1) - S19–S40
-
Narendran P. (2018) Diabetologia. 62(1) 24-27
-
Cherubini V et al. (2020) Diabetologia. 63(8) 1530-1541
-
Ziegler A & Bonifacio E. (2012) Diabetologia 55 1937–43
-
Peters A. (2021) Journal Fam Pract. 70(6S) S47-S52
-
Phillip et al. (2024) ADA & EASD, Consensus Guidelines
-
Larsson HE et al. (2011) Diabetes Care. 34(11) 2347-2352
-
Hekkala AM et al. (2018) Pediatr Diabetes. 19(2) 314-319
-
Jacobsen LM et al. (2022) Diabetes Care. 45(3) 624-633
-
Smith LB et al. (2018) Pediatr Diabetes. 19(5) 1025-1033
-
Insel RA et al. (2015) Diabetes Care. 38(10) 1964-1974
-
McCall AL & Farhy LS. (2013) Minerva Endocrinol. 38(2) 145-163
-
van Belle T, et al. (2011) Physiol Rev. 91(1) 79-118
-
Jacobsen LM et al. (2018) Front Endocrinol (Lausanne) 6,9 70
-
Mayo Clinic. Diabetes symptoms when diabetes symptoms are a concern https //www.mayoclinic.org/diseases-conditions/diabetes/in-depth/diabetes-symptoms/art-20044248 (Accessed 02 May 2024)
-
Gregory et al. (2022) Lancet Diabetes Endocrinol Vol. 10, Issue 10, p741-760
-
Muñoz C, et al. (2019) Clin Diabetes. 37(3) 276-281
-
Regnell SER & Lenmark Å (2017) Diabetologia, Vol. 60, pages 1370–1381
-
Karges B et al. (2021) Diabetes Care. 44(5) 1116-1124
-
Duca LM, et al. (2017) Diabetes Care. 40(9) 1249-1255
-
Harvard health. https://www.health.harvard.edu/a_to_z/type-1-diabetes-mellitus-a-to-z (Accessed 02 May 2024)
-
Cameron FJ et al. (2014). Diabetes Care. 37(6) 1554-1562
-
Birkebaek NH et al. (2022) Lancet Diabetes Endocrinol. 10(11) 786‐794
-
Wolfsdorf et al. (2009) - Pediatric Diabetes, vol. 10(Suppl. 12) 118–133
-
Glaser N et al. (2022) ISPAD clinical practice consensus guidelines, 23(7) 835-856
-
Jiang J et al. RCSB PDB-101 https //pdb101.rcsb.org/global-health/diabetes-mellitus/monitoring/complications (Accessed 02 May 2024)
-
Foster NC, et al. (2019) Diabetes Technol Ther. 21(2) 66-72
-
Katsarou A et al. (2017) Nat Rev Dis Primers. A1063 17016
-
Tiberti et al. (2011) Clin Exp Immunol. 166(3) pp.317-324
-
Cortez et al. (2020) PLoS One. 15(11), pub. Nov 13 e0242049
-
Arvan P, et al. (2012) Cold Spring Harb Perspect Med. 2(8) a007658
-
DiMeglio LA, et al. (2018) Lancet. 391(10138) - 2449-2462
-
Wherrett DK et al. (2015) Diabetes Care 38, pp1975–85
-
Parikka et al. (2012) Diabetologia 55(7)1926-36
-
Krischer et al. (2015) Diabetologia, May 58(5) 980–987
-
Bonifacio E. (2015) Diabetes Care, vol. 38, 2015, pp.989-996
-
Hawa et al. (2013) Diabetes Care, Apr 36(4) pp.908–913
-
Kimbell et al. (2021) BMC Pediatrics vol. 21, Article no. 160
-
Rikos N, et al. (2022) NursRep. 12(3)564-573
-
Schneider J et al. (2023) Diabetologia. 66(12) pp.2387-2388
-
Hummel et al. (2023) Diabetologia, Vol. 66, pp. 1633–1642
-
David MN et al. (2014) Diabetes Care 37(1), pp 9–16
-
Steffes MW et al. (2003) Diabetes Care 26(3), pp832–836
-
Jeyam A et al. (2021) Diabetes Care 44, pp390–398
MAT-NL-2400507 – v1.0 – 06/2024