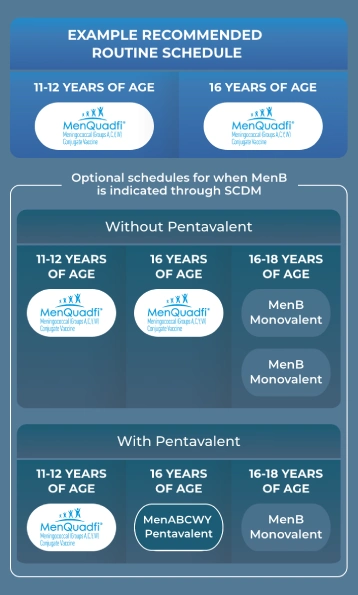
This example ACIP schedule demonstrates how MenQuadfi and stand alone MenB can be an option to complete the ACIP recommendations.3
.webp)
MENQUADFI IS AN OPTION TO COMPLETE THE ROUTINELY RECOMMENDED SCHEDULE
- MenACWY should be administered routinely at 11-12 and 16 years of age3
- MenB is optional through SCDM rather than routinely recommended3
- MenQuadfi comes in a ready-to-use presentation that does not require reconstitution1
MENQUADFI AND A MONOVALENT MENB VACCINE EFFECTIVELY FULFILL YOUR IMD IMMUNIZATION NEEDS
- MenQuadfi and MenB vaccines are fully liquid—no reconstitution required1
- MenB is not routinely recommended in the ACIP vaccination schedule, so stocking both vaccines separately gives you flexibility for MenB vaccination when it’s needed1
IMD=invasive meningococcal disease.
A PENTAVALENT IS AN OPTION WHEN MenACWY AND MenB ARE TO BE GIVEN AT THE SAME VISIT
- Follow-up visits to administer the second dose of MenB are needed 6 months after the pentavalent is administered3
- Because some patients only need a MenACWY vaccine based on the ACIP guidelines, you may still need to stock a quadrivalent vaccine
Important Safety Information
ACIP = Advisory Committee on Immunization Practices; MenABCWY = N meningitidis serogroups A, B, C, W, and Y; MenACWY = N meningitidis serogroups A, C, W, and Y; MenB = meningitis B; SCDM = shared clinical decision making.
MenQuadfi is a registered trademark of Sanofi.
REFERENCES:
1. MenQuadfi [Prescribing Information]. Sanofi.
2. Collins J; ACIP Meningococcal Vaccines Work Group. National Center for Immunization and Respiratory Diseases. Centers for Disease Control and Prevention. Summary of EtR and proposed recommendations for Pfizer’s MenABCWY vaccine. October 25, 2023. Accessed June 26, 2025. https://stacks.cdc.gov/view/cdc/134696
3. Mbaeyi SA, Bozio CH, Duffy J, et al. Meningococcal vaccination: recommendations of the Advisory Committee on Immunization Practices, United States, 2020. MMWR Recomm Rep. 2020;69(9):1-41.
.webp)
.webp)