See subsequent data as to why MenQuadfi and its high rate of seroresponse is a valuable consideration when reviewing your meningococcal vaccine options. Review the full ACIP Schedule.1,2
MenQuadfi elicited an effective seroresponse across all 4 serogroups vs Menveo® and Menactra®1,3*†
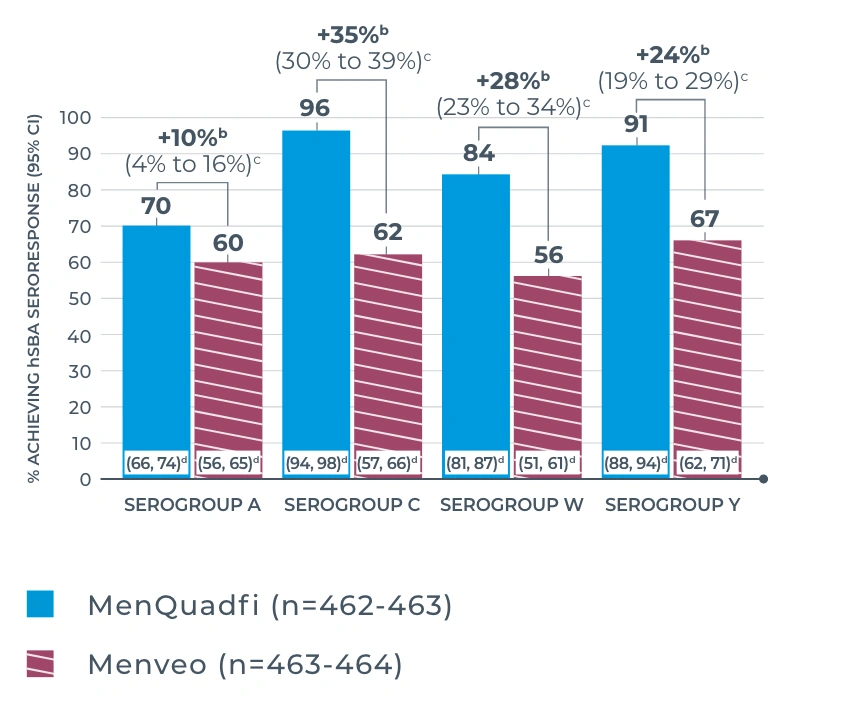
MENQUADFI VS MENVEO
Study design: The immunogenicity and safety of MenQuadfi in adolescents 10 through 17 years of age were evaluated in a randomized, head-to-head clinical trial vs Menveo. Analyses included more than 900 participants. Serum was collected at baseline and 30 days post vaccination to measure antibodies with an hSBA.1
Percentage of subjects achieving vaccine seroresponsea at day 30 (primary endpoint)1
.webp)
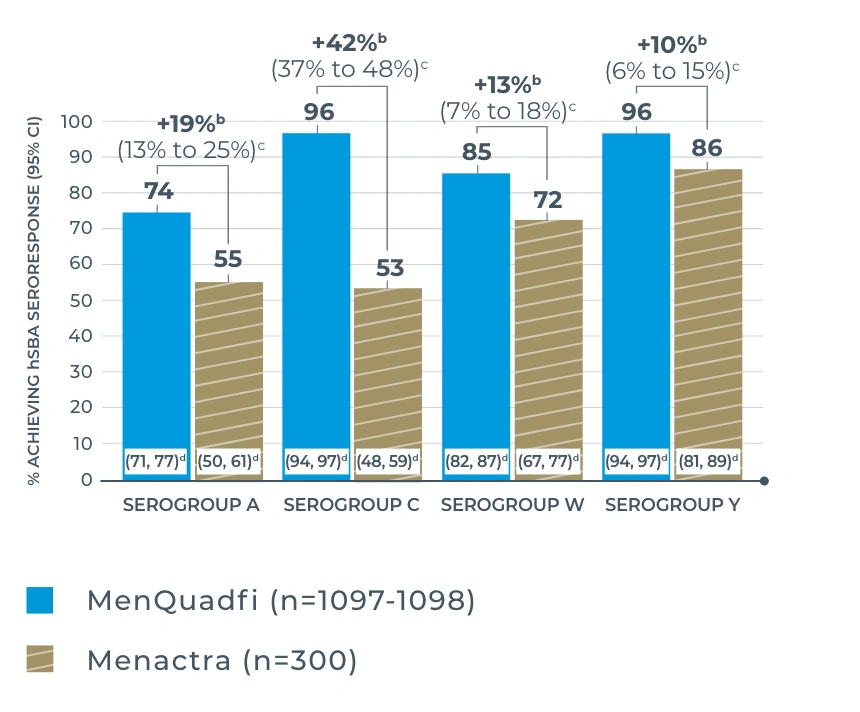
MENQUADFI VS MENACTRA
Study design: The immunogenicity and safety of MenQuadfi were evaluated in a randomized, head-to-head clinical trial vs Menactra that included individuals 10 to 55 years of age (N=3344). Analyses were stratified by age, including a subpopulation consisting of nearly 1400 adolescents 10 through 17 years of age. Serum was collected at baseline and 30 days post vaccination to measure antibodies with an hSBA.1,3,4
Seroresponse rates for MenQuadfi were non-inferior to those of Menactra for all serogroups based on the same non-inferiority criteria defined by the MenQuadfi vs Menveo study.1
Percentage of subjects 10-17 years of age achieving vaccine seroresponsea at day 30 (secondary analysis)2
.webp)
Important Safety Information
ACIP = Advisory Committee on Immunization Practices; CI = confidence interval; hSBA = human serum bactericidal assay; N = number of participants in per-protocol analysis set with valid serology results.
* Menveo (Meningococcal [Groups A, C, Y, and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine).
† Menactra (Meningococcal [Groups A, C, Y, and W-135] Polysaccharide Diphtheria Toxoid Conjugate Vaccine).
a Defined as the proportion of participants with an hSBA pre-vaccination titer <1:8 who achieved a post-vaccination titer ≥1:16, or participants with a pre-vaccination titer ≥1:8 who achieved a post-vaccination titer at least 4-fold greater than the pre-vaccination titer.1
b Percent difference: MenQuadfi minus Menveo or Menactra (95% CI).2
c 95% CI of the difference calculated from the Wilson Score method without continuity correction; the overall non-inferiority would be demonstrated if the lower limit of the 2-sided 95% CI is ≥10% for all 4 serogroups.2
d 95% CI of the single proportion calculated from the exact binomial method.1
MenQuadfi is a registered trademark of Sanofi.
Menactra is a registered trademark of Sanofi, its affiliates, and its subsidiaries.
The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to Sanofi, its affiliates and/or its subsidiaries.
REFERENCES:
1. MenQuadfi [Prescribing Information]. Sanofi.
2. Collins J; ACIP Meningococcal Vaccines Work Group. National Center for Immunization and Respiratory Diseases. Centers for Disease Control and Prevention. Summary of EtR and proposed recommendations for Pfizer’s MenABCWY vaccine. October 25, 2023. Accessed June 26, 2025. https://stacks.cdc.gov/view/cdc/134696
3. Sanofi. Data on File. July 27, 2020.
4. Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a phase III randomized study. Vaccine. 2020;38(33):5194-5201.
.webp)
.webp)