The safety of MenQuadfi has been evaluated in more than 10,000 trial participants 6 weeks and older1
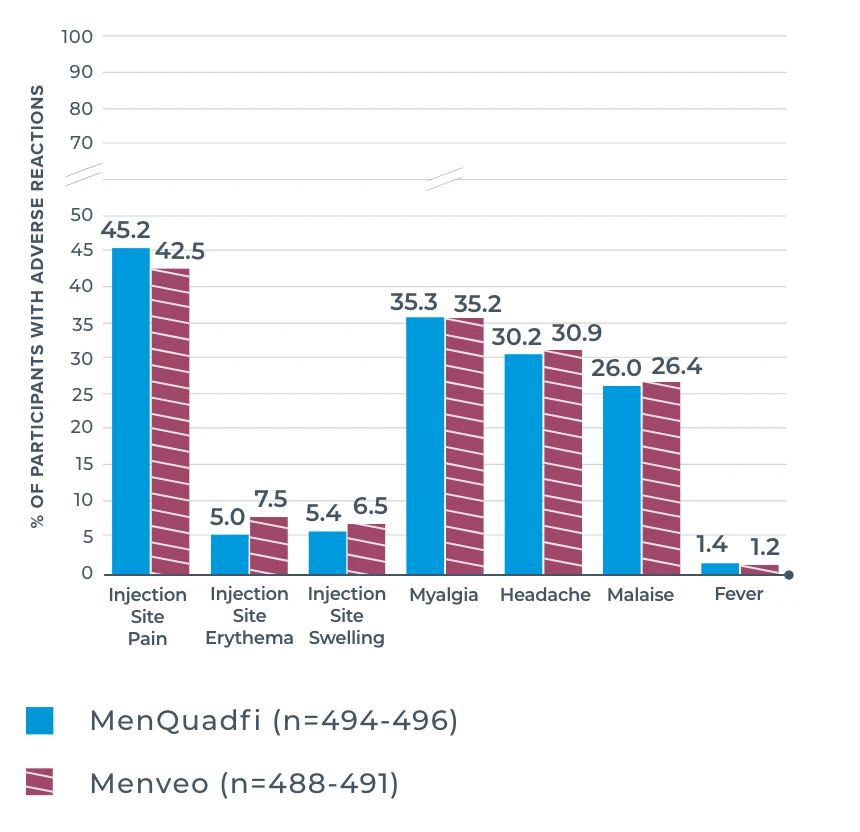
MENQUADFI VS MENVEO
Study design: The immunogenicity and safety of MenQuadfi in adolescents 10 through 17 years of age were evaluated in a randomized, head-to-head clinical trial vs Menveo. Analyses included more than 900 participants. Solicited injection-site and systemic reactions were recorded by participants daily for 7 days following vaccination. All unsolicited adverse events that occurred within 30 days following vaccination were recorded by participants and collected at the next visit, and all serious adverse events were collected for at least 6 months after vaccination.1
Frequency of solicited injection-site reactions and systemic adverse reactions within 7 days after vaccination with MenQuadfi or Menveo1
.webp)
MENQUADFI VS MENACTRA
Study design: The immunogenicity and safety of MenQuadfi were evaluated in a randomized, head-to-head clinical trial vs Menactra that included individuals 10 to 55 years of age (N=3344). Analyses were stratified by age, including a subpopulation consisting of nearly 1400 adolescents 10 through 17 years of age. Solicited injection-site and systemic reactions were recorded by participants daily for 7 days following vaccination. All unsolicited adverse events that occurred within 30 days following vaccination were recorded by participants and collected at the next visit, and all serious adverse events were collected for at least 6 months after vaccination.1-3
Frequency of solicited injection-site reactions and systemic adverse reactions within 7 days after vaccination with MenQuadfi or Menactra1
Important Safety Information
N = number of vaccinated participants with available data for the events listed.
* Menveo (Meningococcal [Groups A, C, Y, and W-135] Oligosaccharide Diphtheria CRM197 Conjugate Vaccine).
† Menactra (Meningococcal [Groups A, C, Y, and W-135] Polysaccharide Diphtheria Toxoid Conjugate Vaccine).
MenQuadfi is a registered trademark of Sanofi.
Menactra is a registered trademark of Sanofi, its affiliates, and its subsidiaries.
The other brands listed are trademarks owned by or licensed to their respective owners and are not owned by or licensed to Sanofi, its affiliates and/or its subsidiaries.
REFERENCES:
1. MenQuadfi [Prescribing Information]. Sanofi.
2. Dhingra MS, Peterson J, Hedrick J, Pan J, Neveu D, Jordanov E. Immunogenicity, safety and inter-lot consistency of a meningococcal conjugate vaccine (MenACYW-TT) in adolescents and adults: a phase III randomized study. Vaccine. 2020;38(33):5194-5201.
3. Sanofi. Data on File. July 27, 2020.
.webp)
.webp)